Self-Organisation
As stated in the famous Aristotle’s quote “The whole is greater than the sum of its parts”, in biological systems the individual components do not necessarily explain the collective behaviour of a group. Collective behaviour is a fundamental scale-crossing principle that underlies the emergence of patterns of a higher order. During development, pattern formation is the process by which initially identical cells acquire different forms and functions. In the lab we explore the role of self-organization as a fundamental property of multicellularity. Therefore, we study cellular mechanisms by which single cells in a multicellular system sense their local environment driving collective behaviours during developmental and regenerative processes. Finally, we develop technologies that cross scales at the spatial, temporal and functional level to bridge the gap between single-cells and organised tissues.
Symmetry Breaking
An important defining step in pattern formation is the moment when initially identical cells in a developing tissue differentiate and lineage segregation is established, and symmetry breaks. Precisely, the symmetry-breaking event occurs when, despite all cells being exposed to a uniform growth-promoting environment, only a fraction becomes activated and differentiates. This process is called symmetry "breaking" because the transitions usually bring the system from a symmetric but disordered and variable state into one or more defined, less variable and asymmetric states (e.g. differentiated states). In the lab we are characterizing the triggering mechanisms of the symmetry-breaking events and how some cells may acquire specific cell fates due to an increased responsiveness to extracellular signals and higher probability of transition.
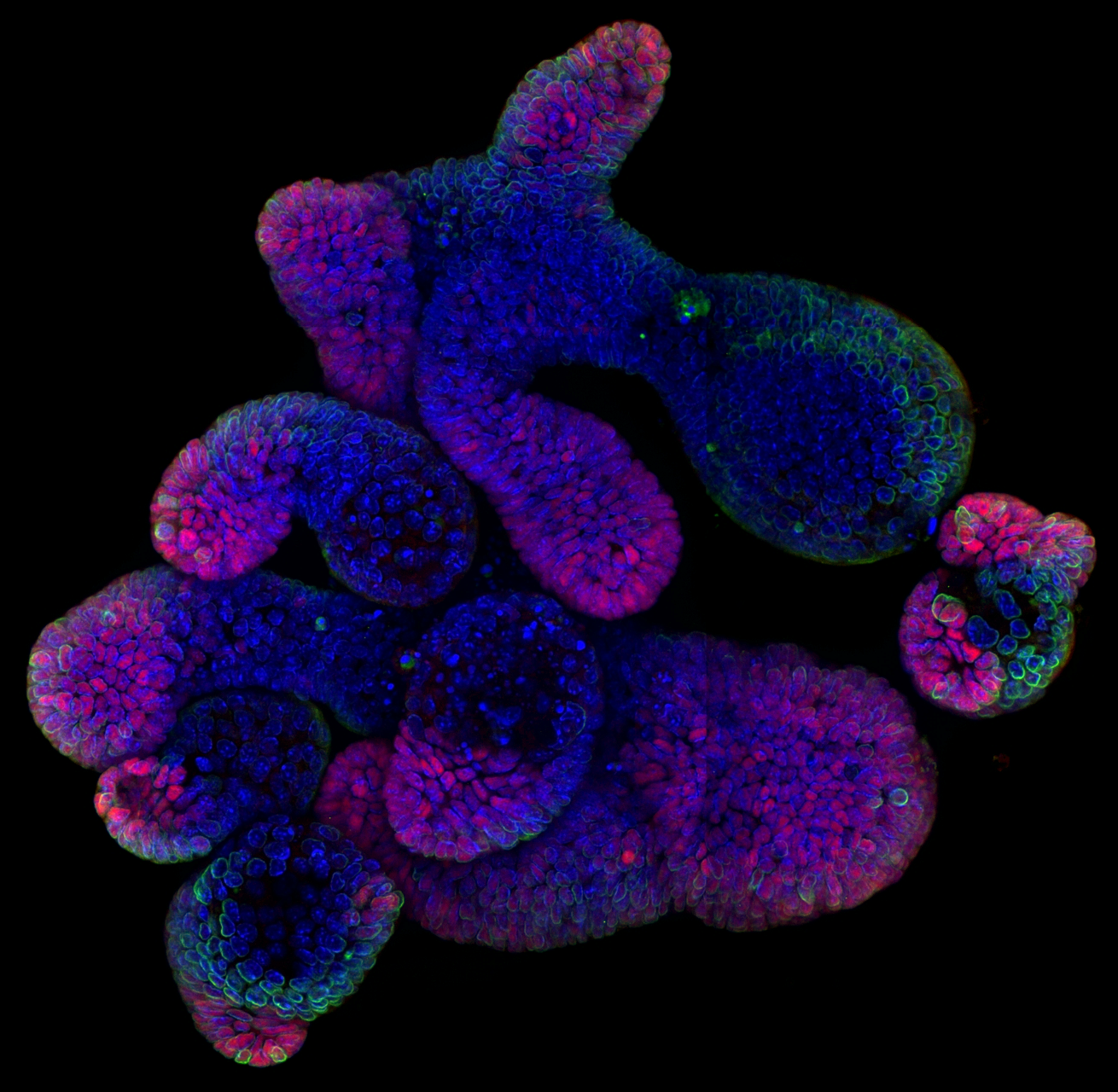
Intestinal Organoid Development
Intestinal organoids are complex three-dimensional structures that mimic cell type composition and tissue organization of the intestine by recapitulating the self-organizing capacity of cell populations derived from a single stem cell. Crucial in this process is a first symmetry-breaking event, in which only a fraction of identical cells in a symmetrical sphere differentiate into Paneth cells, which in turn generates the stem cell niche and leads to asymmetric structures such as crypts and villi. We recently combined a quantitative imaging approach with single-cell gene expression to characterize the development of intestinal organoids from a single cell, showing that their development follows a regeneration process driven by transient Yap1 activation. In the lab we are interested to further understand how single intestinal stem cells exposed to a uniform growth-promoting environment have the intrinsic ability to generate emergent, self-organized behavior resulting in the formation of complex multicellular asymmetric structures.
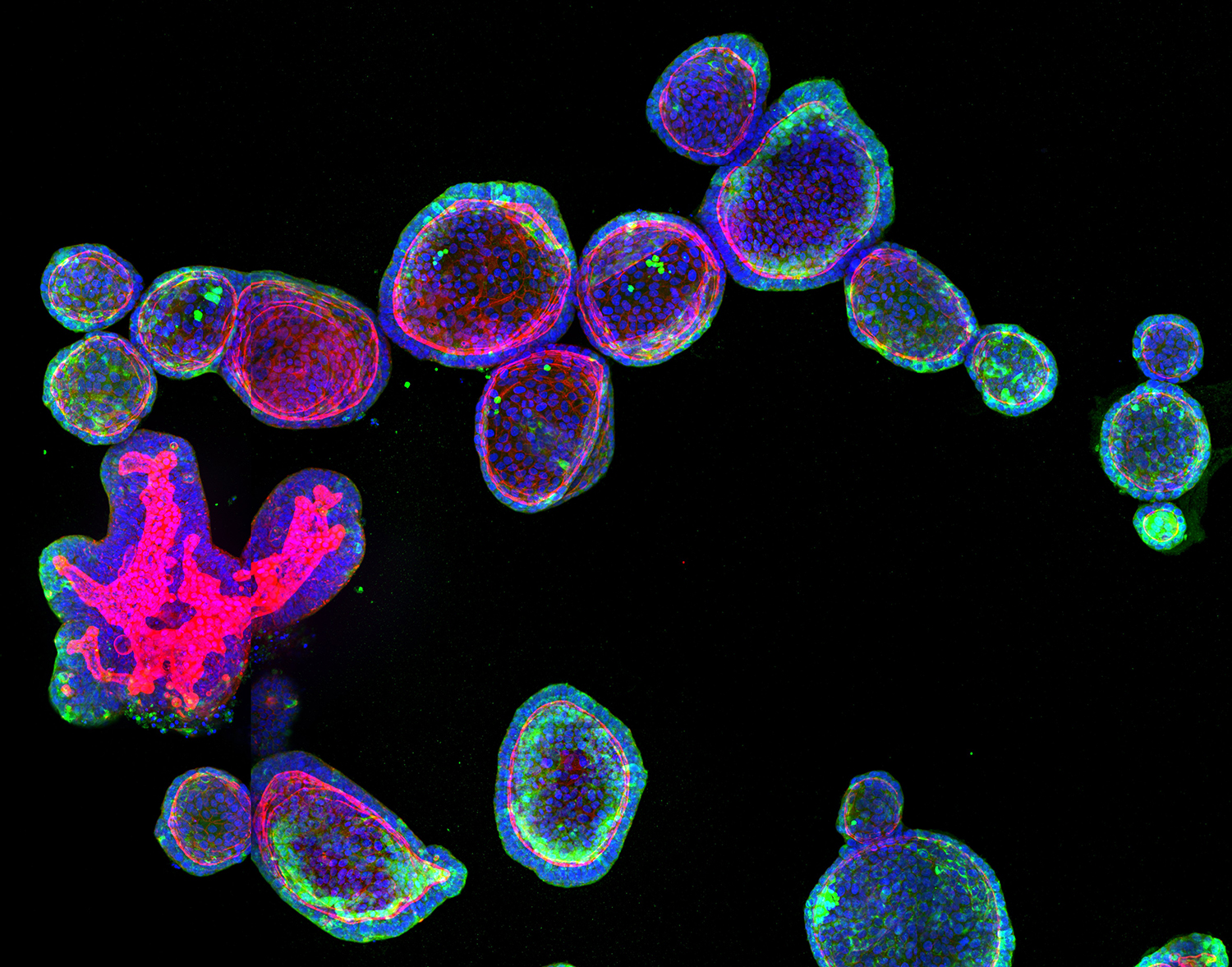
In vitro models of human intestinal regeneration
Mouse intestinal organoids are robust model systems which showcase a regenerative response during the first 48 hours of their development after seeding single cells. This allows laboratories around the world to study a naturally inaccessible and very dynamic process in vitro. Until recently, human biopsy-derived organoids suffered from growth failure after starting from single cells and lacked cell types in their mature state. This made it impossible to study how the injury-induced regeneration is controlled in humans and if the underlying mechanisms are conserved between species. To this end, we develop novel culturing approaches to improve growth conditions of biopsy-derived human organoids and harness their full potential to serve as a model system for intestinal regeneration. We use these improved cultures in combination with high-throughput confocal and long-term lightsheet imaging, as well as sequencing approaches to study how the regenerative response is initiated and stopped at correct timepoints.
Cell-to-cell variability and tissue patterning
Cell-to-cell variability refers to the fact that within a tissue or a population of cells, no two genetically identical cells behave and look identical. Over time, differences between cells arise naturally from the inherent nonlinearity of biochemical reaction networks, a general feature of cells as complex dynamical systems. These initial differences are stabilized by intracellular feedback loops as well as a continual communication between the cell and its external environment. Together, these dynamical feedbacks allow cells to continuously adapt to local changes, amplify initial asynchronies and cell state differences, and ultimately stabilize individual fate outcomes. In the laboratory, we are interested in exploring the role of cell-to-cell variability in cell fate emergence during development. In particular, we are studying the sources of cell-to-cell variability, how initial cell-to-cell variability can lead to robustness in cell fate outcome, and which molecular mechanisms coordinate the simultaneous emergence of cell fates in a developing tissue at the proper proportions, spatial location, and time
Gastruloids as model systems of early development
In the laboratory we are interested in many different self-organized and coordinated events during development. Embryonic stem cells have the intrinsic capacity to follow developmental trajectories in vitro and to give rise to specialised cell types similar to in vivo embryogenesis. Recent studies have demonstrated that they also have the remarkable ability to self-organise into complex structures, mimicking the morphogenetic changes during embryonic development. Specifically, we use gastruloids from mouse embryonic stem cells, to study which events are crucial in order to break symmetry in a coordinated and robust manner, and how this population property emerges.
Mechanosensing mechanisms
The recent development of intestinal organoid technology provides a unique opportunity to quantify morphogenesis and dissect the relationship between morphogenesis and cell fate. Moreover, it allows the determination of the underlying mechano-sensing molecular machineries. More specifically, in the lab we aim to understand the biophysical mechanisms in organoid morphogenesis and find out how tissue mechanics feedback to cell state.
Organoids as disease models
Maintaining tissue homeostasis, via periodical tissue renewal and regenerative processes, requires spatio-temporal coordination of cells to ensure tissue function and integrity. The malfunction of these coordinated behaviours during embryogenesis is the cause of many congenital disorders and their deregulation during adult life in actively proliferating and regenerating tissues, such as the intestine, is the basis of many cancers. In the lab we are interested in building next generation human in vitro disease models that recapitulate the complexity of tissue organisation in health and disease to study temporal processes such as emergence of colorectal cancer.